From The Health Forum NZ @ Facebook
This week Medsafe announced a new form of “active surveillance” for CV V adverse reactions.
This will involve texting a random sample of 10% of V recipients, at various time intervals, soliciting information about reactions.
I am pleased to see this for of surveillance added….while also wondering why we waited for nearly 2 million CV Vs to be administered before acknowledging the need for this.
Having spent five months at the Citizens “coal face” of death and injury, one thing i know for sure…
the “passive” surveillance we have had to date has done an abysmal job of collecting and collating the true extent of injury.
Our roll out started at the end of February and no serious injury data was even collected until 7th of April, and then only incidence of anaphylaxis.
The other serious incidences of heart attacks, strokes, Bels Palsy etc was not monitored until 15th May.
Note the warning that “causal link has not been proven so harm should be treated as a coincidence”
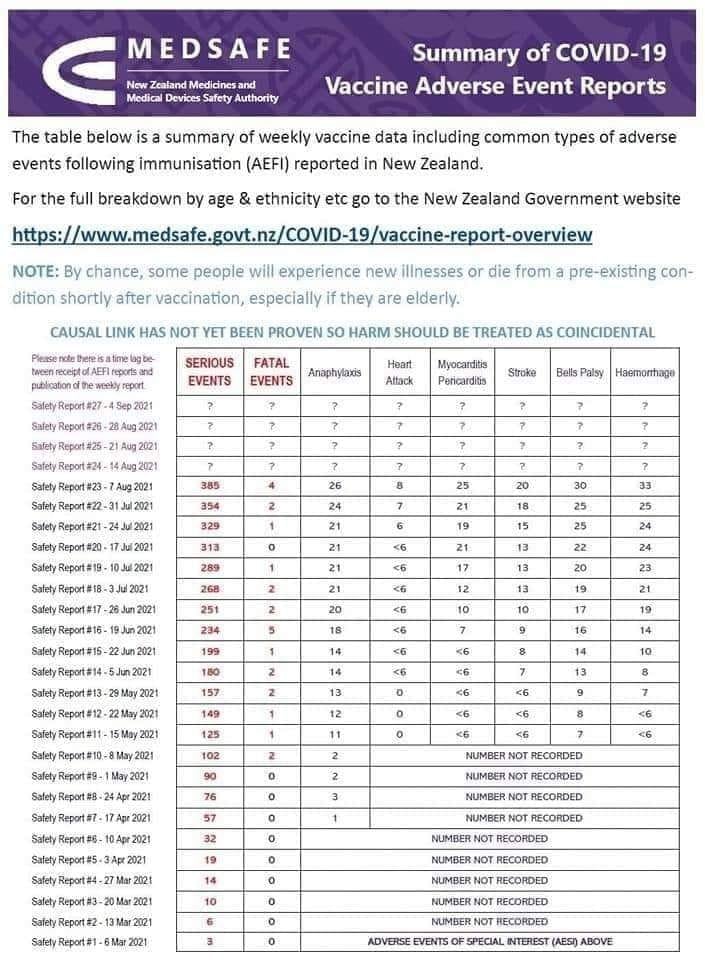
Photo: unsplash.com


You must be logged in to post a comment.